Enzyme-linked immunosorbent assays (ELISAs) remain one of the most powerful tools in modern bioscience, widely used for detecting and quantifying proteins, peptides, antibodies, and hormones. Their broad applicability in diagnostics, research, and drug development makes the ELISA kits a staple in laboratories worldwide. However, the sensitivity, specificity, and reproducibility of ELISA assays hinge on optimized protocols and a comprehensive understanding of kit components and assay variables.
By adhering to protocols and optimization strategies, researchers can unlock the full potential of ELISA kits, ensuring data integrity and enhancing the reliability of their analytical outcomes.
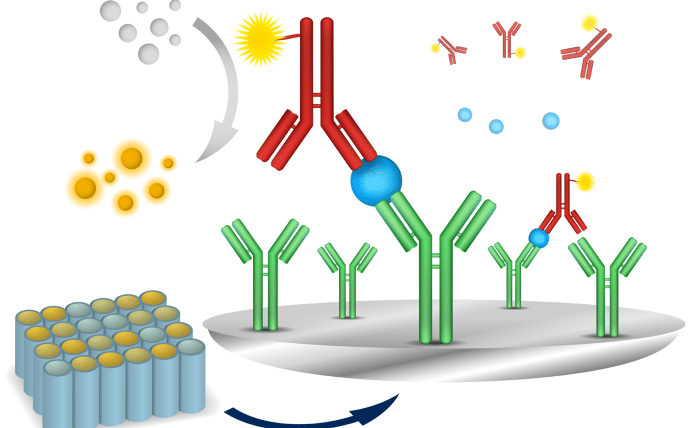
Understanding ELISA kits: components and formats
Commercial ELISA kits are designed to simplify assay development, offering pre-coated plates, standardized reagents, and validated ELISA protocols. Standard formats include direct, indirect, sandwich, and competitive ELISAs, each suited to different analyte types and concentration ranges.
- Direct ELISA uses a labeled primary antibody and is ideal for detecting high-abundance antigens. It offers a shorter protocol and reduced background but typically lower sensitivity.
- Indirect ELISA introduces a labeled secondary antibody, enhancing sensitivity through signal amplification. This format is particularly useful for detecting antibodies in serum or plasma.
- Sandwich ELISA, the most sensitive format, employs both capture and detection antibodies, making it suitable for low-abundance targets such as cytokines or biomarkers.
- Competitive ELISA is best suited for small antigens or analytes with single antibody binding sites and is frequently used in hormone and drug screening.
Understanding the kit’s design and the biochemical nature of the target analyte is essential for proper assay planning. Key components, such as capture and detection antibodies, enzyme conjugates, standards, and substrates, must be validated for compatibility and used within their defined operational ranges to avoid non-specific signals or signal loss.
Pre-assay considerations
Before initiating any ELISA, researchers should consider the following:
Sample preparation
Sample matrix complexity significantly influences ELISA performance. Biological samples like serum, plasma, or cell lysates may introduce interfering substances. Dilution, centrifugation, and proper storage minimize variability and preserve antigen integrity. Using protease inhibitors or detergent-free lysis buffers may be necessary, depending on the target.
Reagent storage and handling
Strict adherence to storage guidelines is essential. Enzyme conjugates and antibodies are often temperature-sensitive. They should be stored as per the manufacturer’s guidelines, typically at cold conditions (-20°C or 4°C). Repeated freeze-thaw cycles should be avoided, and reagents must be equilibrated to room temperature before use to prevent condensation effects.
Plate handling
To ensure consistent coating and minimize pipetting errors, touching the interior surfaces of wells should be avoided, and pipetting should be performed carefully. Calibrated, low-retention tips can help reduce pipetting errors. To mitigate plate edge effects, caused by uneven incubation conditions, edge wells can be used as buffer-only controls.
Best practices during the assay
Standard curve optimization: Generating an accurate standard curve is foundational for quantitative ELISA. Standards should be freshly prepared and cover a relevant concentration range. Logarithmic plotting typically yields a better fit for non-linear standard curves.
Consistent timing and incubation: Incubation times and temperatures must be consistent across wells and plates. Slight deviations can lead to signal drift, particularly in colorimetric assays. Automated plate washers and incubators can help maintain uniformity.
Washing steps: Improper washing can lead to a high background or poor signal. Using recommended volumes of wash buffer and wash cycles is important. Residual wash buffer can dilute subsequent reagents.
Substrate reaction monitoring: The enzyme-substrate reaction time should be optimized based on signal-to-noise ratio. Overdevelopment can saturate the signal, while underdevelopment can lead to weak signals. Reading the plate within the optimal time window is essential.
For instance, chromogenic substrates like TMB (3,3′,5,5′-Tetramethylbenzidine) require careful monitoring.
Post-assay validation
Quality controls: Positive and negative controls should be included in each run. They assure assay validity and highlight inconsistencies across plates or batches. Monitoring control values over time also supports long-term assay stability.
Data interpretation: Using appropriate software to fit standard curves is important to interpolate sample concentrations. A coefficient of determination (R²) above 0.99 is generally considered indicative of a reliable curve.
Troubleshooting
In ELISA, several common problems can be resolved with proper technique and attention to detail. Inadequate washing, contaminated reagents, or nonspecific binding often cause a high background. It can be reduced by ensuring thorough washing, using freshly prepared buffers, and applying appropriate blocking steps.
Low signal may occur if reagents are expired or stored improperly, antibody concentrations are incorrect, or target antigens are degraded. These issues can be addressed by verifying reagent quality, optimizing antibody dilutions, and ensuring proper sample handling.
High variability is typically introduced through inconsistent pipetting, edge effects, or uneven incubation. It can be minimized by calibrating pipettes regularly, using plate mapping strategies, and randomizing sample placement to maintain uniform assay conditions.
Standardization and reproducibility
To ensure reliable ELISA results, strict adherence to standardized protocols is essential. Inter-laboratory studies have demonstrated that assay reproducibility improves significantly when procedures are harmonized, and technical variation is minimized. Automation of the major steps, such as pipetting and washing, reduces human error and enhances consistency across runs.
Routine pipette calibration, use of certified reference materials, and thorough documentation support good laboratory practice (GLP) and align with FAIR data principles. These measures not only improve reproducibility but also strengthen data transparency and credibility in scientific research.
ELISA kits are powerful tools, but achieving reliable results requires deliberate optimization. From selecting the correct format to fine-tuning incubation steps and data analysis, each phase of the assay process offers opportunities for improvement. By applying the best practices outlined here and using validated ELISA kits from reputable manufacturers, researchers can significantly improve data quality and reproducibility. As ELISA remains one of the most trusted immunoassays, mastering its protocols is essential for reliable outcomes in diagnostics, biomarker discovery, and beyond.




